Carbon-Carbon Bond Formation: How to Extend the Chain by One Carbon
For A-level Chemistry, one of the key synthetic skills you’re expected to master is carbon–carbon bond formation, in other words, how to make a molecule longer by adding one extra carbon atom. There are three core reactions you need to know. Each involves a different functional group, different reagents, and a different reaction mechanism. Let’s break them down clearly.
1. Extending the Chain Using Halogenoalkanes
Reagents: Potassium cyanide (KCN) in ethanol
Mechanism: Nucleophilic substitution (SN2)
Functional group involved: Halogenoalkanes (bromoalkanes, chloroalkanes, etc.)
In this reaction, the nucleophile is the cyanide ion (CN⁻). The cyanide ion attacks the δ⁺ carbon attached to the halogen and substitutes the halide ion.
Why this extends the chain:
The CN⁻ adds a –C≡N group, which introduces one additional carbon atom into the molecule. You can later convert the nitrile into a range of other functional groups (e.g., carboxylic acids or amines), making this a very useful synthetic step.
General reaction:
R–X + CN⁻ → R–C≡N + X⁻
Mechanism
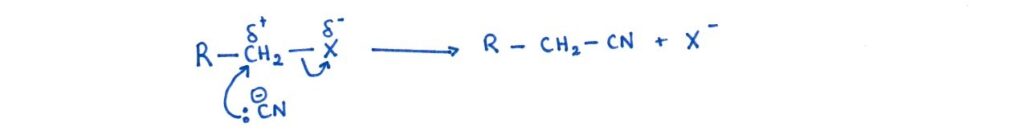
2. Extending the Chain Using Carbonyl Compounds (Ketones and Aldehydes)
Reagents: Sodium cyanide (NaCN, aqueous) and H⁺ (aq)
Mechanism: Nucleophilic addition
Functional group involved: Aldehydes and ketones
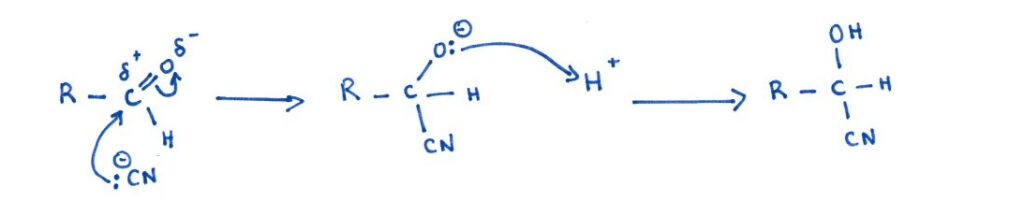
Here, the cyanide ion adds across the C=O double bond of an aldehyde or ketone. The attack of CN⁻ on the electrophilic carbon forms an intermediate, which is then protonated to give a hydroxynitrile (cyanohydrin).
This reaction involves an heterolytic fission. Heterolytic, because one bonded atom, here O receive both electrons and fission because a covalent bond is broken.
General reaction:
Carbonyl + HCN → Hydroxynitrile
Why this extends the chain:
Just like with halogenoalkanes, the CN group adds one extra carbon to the molecule. This method is especially useful when you want to extend chains while also introducing a new chiral centre (if the carbonyl is asymmetric).
Mechanism:
3. Extending the Chain Using Friedel–Crafts Reactions
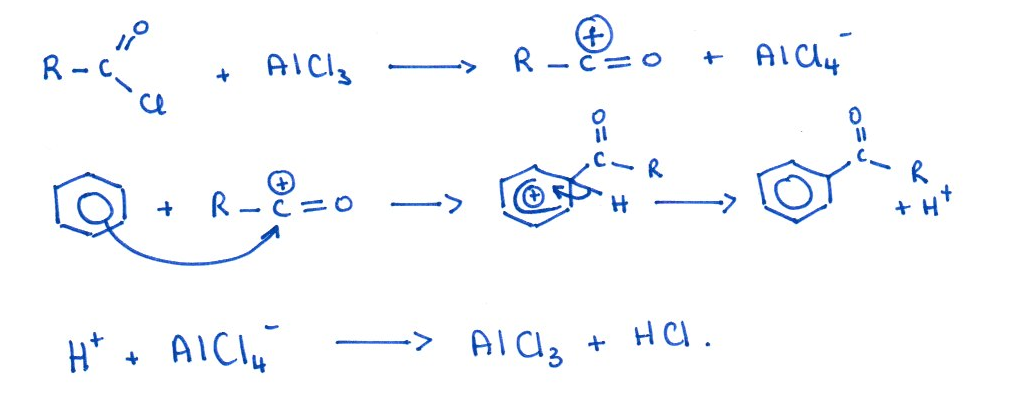
Acylation: Acyl chloride + AlCl₃ (halogen carrier). This reaction adds an acyl group (–COR).
Alkylation: Halogenoalkane + AlCl₃. This reaction adds an alkyl chain (e.g. –CH₃, –CH₂CH₃).
Mechanism: Electrophilic substitution
Functional group involved: Arenes (e.g. benzene)
Friedel–Crafts reactions allow you to add carbon-containing groups directly onto an aromatic ring.
Mechanism:
4. Subsequent Reactions
In exam questions, the nitrile group introduced by these reactions is often taken further, either reduced to an amine group ( –CH₂NH₂) or hydrolysed to form a carboxylic acid, so be prepared to apply these follow-up steps in synthetic pathways. To learn these reactions in detail, read the next article: the reactions of nitrile group. You might also want to check out how to form halogenoalkanes from alcohols, or how to perform alcohol oxidation to form carbonyl groups.
Any questions, you can contact me to book a lesson.
Pingback: Reactions of Nitrile Group - Chemistry Web Tutor