Oxidation of Alcohols
Oxidation of alcohols is a fundamental reaction in A-level Chemistry and is commonly carried out using acidified potassium dichromate (K₂Cr₂O₇ / H₂SO₄). Depending on the type of alcohol, oxidation can produce a range of functional groups, including aldehydes, ketones, and carboxylic acids; however, tertiary alcohols do not undergo oxidation under these conditions. This makes the reaction particularly useful for identifying the type of alcohol present
1. Identifying Alcohol Types
Before predicting products, it’s important to know whether your alcohol is primary, secondary, or tertiary. What does this mean?
- Primary alcohols (1°) — the –OH group is attached to a carbon bonded to one other carbon.
- Secondary alcohols (2°) — the –OH group is attached to a carbon bonded to two other carbons.
- Tertiary alcohols (3°) — the –OH group is attached to a carbon bonded to three other carbons.
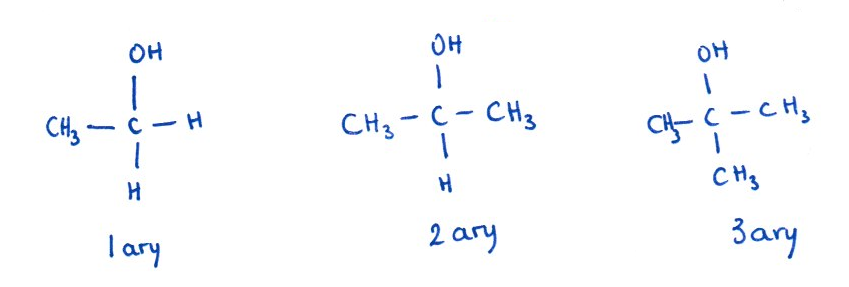
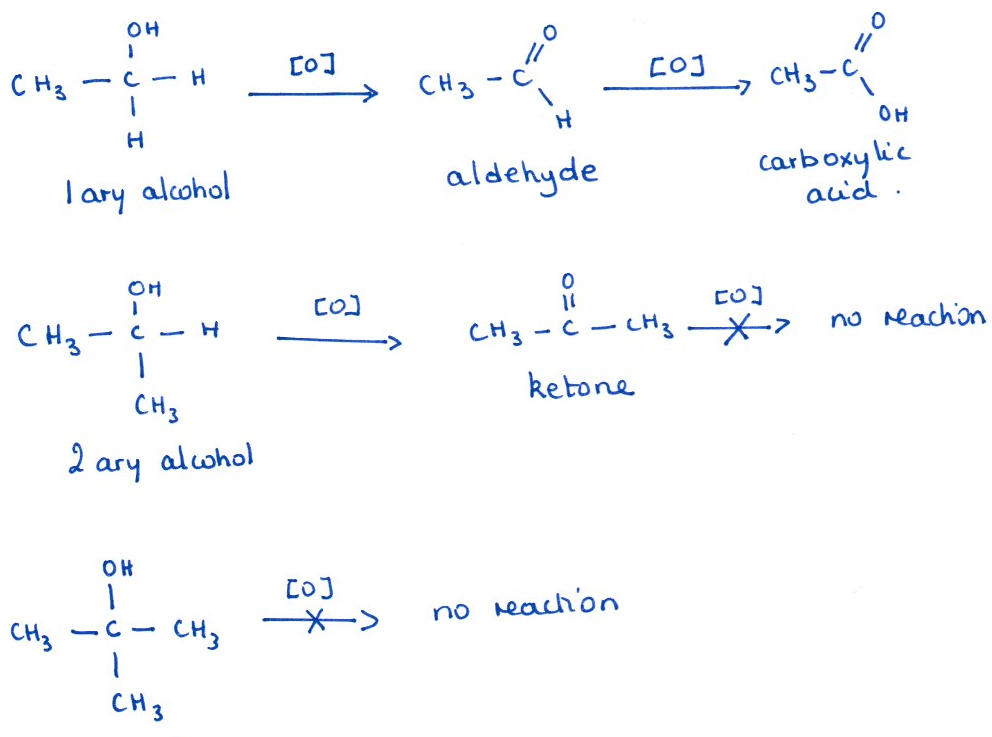
Key point: Oxidation requires a hydrogen atom on the carbon bearing the –OH group. Primary alcohols have two such hydrogens, so they can be oxidised first to an aldehyde and then further to a carboxylic acid. Secondary alcohols have only one hydrogen on the –OH carbon, so they are oxidised to ketones. Tertiary alcohols have no hydrogen on the –OH carbon, so they do not undergo oxidation under these conditions.
2. Reaction Conditions to Control the Oxidation
As discussed above, primary alcohols can be oxidised in two stages: first to an aldehyde, and then further to a carboxylic acid. By adjusting the reaction conditions, it is possible to control the process and selectively obtain either the aldehyde or the carboxylic acid.
- Full oxidation (to carboxylic acid): Reflux the primary alcohol with an excess of acidified potassium dichromate. The constant heating ensures that the aldehyde formed initially remains in contact with the oxidising agent and is further oxidised to the carboxylic acid.
- Partial oxidation (stop at aldehyde): Use an excess of alcohol and distill the primary alcohol with acidified potassium dichromate. As the aldehyde forms, it evaporates and is collected by distillation, removing it from the reaction mixture and preventing further oxidation.
Key point: Distillation exploits the lower boiling point of aldehydes compared to alcohols, allowing the aldehyde to be separated as it forms, so the reaction can be stopped at this stage instead of proceeding all the way to the carboxylic acid. This works because of the difference in intermolecular bonding between the alcohol and the aldehyde:
- Aldehydes (–CHO) have only a polar C=O group, which allows dipole-dipole interactions but not hydrogen bonding between molecules, so they boil at a lower temperature than the alcohol.
- Alcohols can hydrogen bond, aldehydes cannot (effectively). The –OH group in alcohols can form strong intermolecular hydrogen bonds, so more energy is required to break them, which consequently raises their boiling points.
3. Balanced Equations
The common oxidising agent for alcohols is an acidified solution of potassium or sodium dichromate(VI), typically prepared with dilute sulfuric acid. During the oxidation process, the dichromate(VI) ions (orange in solution) are reduced to chromium(III) ions, which give the solution a green colour. In organic chemistry, the oxidising agent is often represented simply by [O], rather than writing out the dichromate half-equation each time.
Primary alcohol to aldehyde (partial oxidation):
CH₃CH₂OH + [O] → CH₃CHO + H₂O
Aldehyde to carboxylic acid:
CH₃CHO + [O] → CH₃COOH
Primary alcohol to carboxylic acid:
CH₃CH₂OH + 2[O] → CH₃COOH + H₂O
Secondary alcohol to ketone:
CH₃CHOHCH₃ + [O] → CH₃COCH₃ + H₂O
Tertiary alcohol:
No reaction under these conditions.
4. Observing the Reaction: Colour Change
Acidified potassium dichromate is an orange solution (Cr₂O₇²⁻). During oxidation:
- The Cr₂O₇²⁻ ions are reduced to Cr³⁺, which is green.
- So you can monitor the reaction visually:
Orange → Green = oxidation is occurring.
Because this reaction is accompanied by a distinctive colour change, this is particularly useful in practical work: primary and secondary alcohols turn the solution from orange to green, while tertiary alcohols show no colour change, allowing them to be identified easily.
If you have any questions or if you want to practice this topic with past papers, contact me below.